Omega-3 fatty acid
| Types of fats in food |
|---|
|
| See also |
|
n−3 fatty acids (popularly referred to as ω−3 fatty acids or omega-3 fatty acids) are a family of unsaturated fatty acids that have in common a final carbon–carbon double bond in the n−3 position; that is, the third bond from the methyl end of the fatty acid.
Nutritionally important n−3 fatty acids include α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), all of which are polyunsaturated. The human body cannot synthesize n−3 fatty acids de novo, but it can form "long chain" 20-carbon unsaturated n−3 fatty acids (like EPA) and 22-carbon unsaturated n−3 fatty acids (like DHA) from the "short chain" eighteen-carbon n−3 fatty acid α-linolenic acid. The short chain n−3 fatty acids are converted to long chain forms (EPA, DHA) with an efficiency of approximately 5%[1][2] in men, and at a greater percentage in women.[3]
These conversions occur competitively with n−6 fatty acids, which are essential closely related chemical analogues that are derived from linoleic acid. Both the n−3 α-linolenic acid and n−6 linoleic acid are essential nutrients which must be obtained from food. Synthesis of the longer n−3 fatty acids from linolenic acid within the body is competitively slowed by the n−6 analogues. Thus accumulation of long-chain n−3 fatty acids in tissues is more effective when they are obtained directly from food or when competing amounts of n−6 analogs do not greatly exceed the amounts of n−3.
Contents |
History
Although omega-3 fatty acids have been known as essential to normal growth and health since the 1930s, awareness of their health benefits has dramatically increased in the past few years.[4] New versions of ethyl esterized omega-3 fatty acids, such as E-EPA and combinations of E-EPA and E-DHA, have drawn attention as highly purified and more effective products than the traditional ones. In the United States, these novel versions are often sold as prescription medications, such as Lovaza. In the European Union, they are available as dietary supplements.
The health benefits of the long-chain omega-3 fatty acids — DHA and EPA omega-3 — are the best known. These benefits were discovered in the 1970s by researchers studying the Greenland Inuit Tribe. The Greenland Inuit people consumed large amounts of fat from seafood, but displayed virtually no cardiovascular disease. The high level of omega-3 fatty acids consumed by the Inuit reduced triglycerides, heart rate, blood pressure, and atherosclerosis.
On September 8, 2004, the U.S. Food and Drug Administration gave "qualified health claim" status to EPA and DHA n−3 fatty acids, stating that "supportive but not conclusive research shows that consumption of EPA and DHA [n−3] fatty acids may reduce the risk of coronary heart disease."[5] This updated and modified their health risk advice letter of 2001 (see below). Currently, regulatory agencies do not accept that there is sufficient evidence for any of the other suggested benefits of DHA and EPA other than for cardiovascular health, and further claims should be treated with caution.
The Canadian Government has recognized the importance of DHA omega-3 and permits the following biological role claim for DHA: "DHA, an omega-3 fatty acid, supports the normal development of the brain, eyes and nerves."[6]
Chemistry
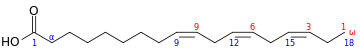
The term n−3 (also called ω−3 or omega-3) signifies that the first double bond exists as the third carbon-carbon bond from the terminal methyl end (n) of the carbon chain.
n−3 fatty acids which are important in human nutrition are: α-linolenic acid (18:3, n−3; ALA), eicosapentaenoic acid (20:5, n−3; EPA), and docosahexaenoic acid (22:6, n−3; DHA). These three polyunsaturates have either 3, 5 or 6 double bonds in a carbon chain of 18, 20 or 22 carbon atoms, respectively. All double bonds are in the cis-configuration; in other words, the two hydrogen atoms are on the same side of the double bond.
Most naturally-produced fatty acids (created or transformed in animalia or plant cells with an even number of carbon in chains) are in cis-configuration where they are more easily transformable. The trans-configuration results in much more stable chains that are very difficult to further break or transform, forming longer chains that aggregate in tissues and lacking the necessary hydrophilic properties. This trans-configuration can be the result of the transformation in alkaline solutions, or of the action of some bacterias that are shortening the carbonic chains. Natural transforms in plant or animal cells more rarely affect the last n−3 group itself. However, n−3 compounds are still more fragile than n−6 because the last double bond is geometrically and electrically more exposed, notably in the natural cis configuration.
Iodine can add to double bonds of docosahexaenoic acid and arachidonic acid forming iodolipids and making them less reactive to free oxygen radicals.[7][8][9][10]
List of n−3 fatty acids
This table lists several different names for the most common n−3 fatty acids found in nature.
| Common name | Lipid name | Chemical name |
|---|---|---|
| n/a | 16:3 (n−3) | all-cis-7,10,13-hexadecatrienoic acid |
| α-Linolenic acid (ALA) | 18:3 (n−3) | all-cis-9,12,15-octadecatrienoic acid |
| Stearidonic acid (SDA) | 18:4 (n−3) | all-cis-6,9,12,15-octadecatetraenoic acid |
| Eicosatrienoic acid (ETE) | 20:3 (n−3) | all-cis-11,14,17-eicosatrienoic acid |
| Eicosatetraenoic acid (ETA) | 20:4 (n−3) | all-cis-8,11,14,17-eicosatetraenoic acid |
| Eicosapentaenoic acid (EPA) | 20:5 (n−3) | all-cis-5,8,11,14,17-eicosapentaenoic acid |
| Docosapentaenoic acid (DPA), Clupanodonic acid |
22:5 (n−3) | all-cis-7,10,13,16,19-docosapentaenoic acid |
| Docosahexaenoic acid (DHA) | 22:6 (n−3) | all-cis-4,7,10,13,16,19-docosahexaenoic acid |
| Tetracosapentaenoic acid | 24:5 (n−3) | all-cis-9,12,15,18,21-tetracosapentaenoic acid |
| Tetracosahexaenoic acid (Nisinic acid) | 24:6 (n−3) | all-cis-6,9,12,15,18,21-tetracosahexaenoic acid |
Biological significance
- The biological effects of the n−3 are largely mediated by their interactions with the n−6 fatty acids; see Essential fatty acid interactions for detail.
A 1992 article by biochemist William E.M. Lands[11] provides an overview of the research into n−3 fatty acids, and is the basis of this section.
The 'essential' fatty acids were given their name when researchers found that they were essential to normal growth in young children and animals. (Note that the modern definition of 'essential' is more strict.) A small amount of n−3 in the diet (~1% of total calories) enabled normal growth, and increasing the amount had little to no additional effect on growth.
Likewise, researchers found that n−6 fatty acids (such as γ-linolenic acid and arachidonic acid) play a similar role in normal growth. However, they also found that n−6 was "better" at supporting dermal integrity, renal function, and parturition. These preliminary findings led researchers to concentrate their studies on n−6, and it was only in recent decades that n−3 has become of interest.
In 1963 it was discovered that the n−6 arachidonic acid was converted by the body into pro-inflammatory agents called prostaglandins. By 1979 more of what are now known as eicosanoids were discovered: thromboxanes, prostacyclins and the leukotrienes. The eicosanoids, which have important biological functions, typically have a short active lifetime in the body, starting with synthesis from fatty acids and ending with metabolism by enzymes. However, if the rate of synthesis exceeds the rate of metabolism, the excess eicosanoids may have deleterious effects. Researchers found that n−3 is also converted into eicosanoids, but at a much slower rate. Eicosanoids made from n−3 fats are often referred to as anti-inflammatory, but in fact they are just less pro-inflammatory than those made from n−6 fats. If both n−3 and n−6 are present, they will "compete" to be transformed, so the ratio of n−3:n−6 directly affects the type of eicosanoids that are produced.
This competition was recognized as important when it was found that thromboxane is a factor in the clumping of platelets, which leads to thrombosis. The leukotrienes were similarly found to be important in immune/inflammatory-system response, and therefore relevant to arthritis, lupus, and asthma. These discoveries led to greater interest in finding ways to control the synthesis of n−6 eicosanoids. The simplest way would be by consuming more n−3 and fewer n−6 fatty acids.
In 1982 Dr. Charles Serhan's group at Harvard discovered that the omega-3 fatty acid EPA forms in the body potent antiinflamatory nanomolecules, called resolvins. Later another team found that omega-3s also turn into other antiinflammatory molecules called omega-3-oxylipins, which partly explain the versatile health effects of fish oil.[12]
Health benefits
The 18 carbon α-linolenic acid has not been shown to have the same cardiovascular benefits as DHA or EPA.[13] Currently there are many products on the market which claim to contain health promoting 'omega 3', but contain only α-linolenic acid (ALA), not EPA or DHA. These products contain mainly higher plant oils and must be converted by the body to create DHA and therefore considered less efficient. DHA and EPA are made by microalgae that live in seawater. These are then consumed by fish and accumulate to high levels in their internal organs. If a person has ethical concerns about killing fish, or is concerned about mercury and oceanborne contaminants in fish, DHA can be produced directly from microalgae as a vegetarian source. People with certain circulatory problems, such as varicose veins, benefit from such supplements containing EPA and DHA which stimulate blood circulation, increase the breakdown of fibrin, a compound involved in clot and scar formation, and additionally have been shown to reduce blood pressure.[14][15] There is strong scientific evidence that n−3 fatty acids reduce blood triglyceride levels[16][17][18][19] and regular intake reduces the risk of secondary and primary heart attack.[20][21][22][23]
Some benefits have been reported in conditions such as rheumatoid arthritis[24][25] and cardiac arrhythmias.[26][27][28]
There is preliminary evidence that n-3 fatty acids supplementation might be helpful in cases of depression[29][30] and anxiety.[31][32] Studies report highly significant improvement from n-3 fatty acids supplementation alone and in conjunction with medication.[33] The New York Times reports that at least one study, however, has found no connection between depression in heart patients and supplements containing n-3 fatty acids.[34]
Some research suggests that fish oil intake may reduce the risk of ischemic and thrombotic stroke.[35][36][37] However, very large amounts may actually increase the risk of hemorrhagic stroke (see below). Lower amounts are not related to this risk,[37] 3 grams of total EPA/DHA daily are considered safe with no increased risk of bleeding involved[38] and many studies used substantially higher doses without major side effects (for example: 4.4 grams EPA/2.2 grams DHA in 2003 study).[29] There is evidence that the botanical sources of n−3 do not result in the health benefits derived from wild fish sources.[39]
Cancer prevention
Several studies report possible anti-cancer effects of n−3 fatty acids (particularly breast, colon, and prostate cancer).[40][41][42] Omega-3 fatty acids reduced prostate tumor growth, slowed histopathological progression, and increased survival.[43] Among n-3 fatty acids [omega-3], neither long-chain nor short-chain forms were consistently associated with breast cancer risk. High levels of docosahexaenoic acid, however, the most abundant n-3 PUFA [omega-3] in erythrocyte membranes, were associated with a reduced risk of breast cancer.[44] A 2006 report in the Journal of the American Medical Association concluded that their review of literature covering cohorts from many countries with a wide variety of demographic concluded that there was no link between n−3 fatty acids and cancer.[45] This is similar to the findings of a review by the British Medical Journal of studies up to February 2002 that failed to find clear effects of long and shorter chain n−3 fats on total mortality, combined cardiovascular events and cancer.[46]
A 2007 systematic review of n-3 fatty acids and cachexia found evidence that oral n-3 fatty acid supplements benefit cancer patients, improving appetite, weight and quality of life.[47] A 2009 trial found that a supplement of eicosapentaenoic acid helped cancer patients retain muscle mass.[48]
Cardiovascular disease prevention
In 1999, the GISSI-Prevenzione Investigators reported in the Lancet, the results of major clinical study in 11,324 patients with a recent myocardial infarction. Treatment 1 gram per day of n−3 fatty acids reduced the occurrence of death, cardiovascular death and sudden cardiac death by 20%, 30% and 45% respectively.[49] These beneficial effects were seen already from three months onwards.[50]
In April 2006, a team led by Lee Hooper at the University of East Anglia in Norwich, UK, published a review of almost 100 separate studies into n−3 fatty acids, found in abundance in oily fish. It concluded that they do not have a significant protective effect against cardiovascular disease.[51] This meta-analysis was controversial and stands in stark contrast with two different reviews also performed in 2006 by the American Journal of Clinical Nutrition[52] and a second JAMA review[53] that both indicated decreases in total mortality and cardiovascular incidents (i.e. myocardial infarctions) associated with the regular consumption of fish and fish oil supplements.
Several studies published in 2007 have been more positive. In the March 2007 edition of the journal Atherosclerosis, 81 Japanese men with unhealthy blood sugar levels were randomly assigned to receive 1800 mg daily of eicosapentaenoic acid (EPA) with the other half being a control group. The thickness of the carotid arteries and certain measures of blood flow were measured before and after supplementation. This went on for approximately two years. A total of 60 patients (30 in the E-EPA group and 30 in the control group) completed the study. Those given the EPA had a statistically significant decrease in the thickness of the carotid arteries along with improvement in blood flow. The authors indicated that this was the first demonstration that administration of purified EPA improves the thickness of carotid arteries along with improving blood flow in patients with unhealthy blood sugar levels.[54]
In another study published in the American Journal of Health System Pharmacy March 2007, patients with high triglycerides and poor coronary artery health were given 4 grams a day of a combination of EPA and DHA along with some monounsaturated fatty acids. Those patients with very unhealthy triglyceride levels (above 500 mg/dl) reduced their triglycerides on average 45% and their VLDL cholesterol by more than 50%. VLDL is a bad type of cholesterol and elevated triglycerides can also be deleterious for cardiovascular health.[55]
Another study on the benefits of EPA was published in The Lancet in March 2007. This study involved over 18,000 patients with unhealthy cholesterol levels. The patients were randomly assigned to receive either 1,800 mg a day of E-EPA with a statin drug or a statin drug alone. The trial went on for a total of five years. It was found at the end of the study those patients in the E-EPA group had superior cardiovascular function. Non-fatal coronary events were also significantly reduced in the E-EPA group. The authors concluded that EPA is a promising treatment for prevention of major coronary events, especially non-fatal coronary events.[56]
Similar to those who follow a Mediterranean diet, Arctic-dwelling Inuit - who consume high amounts of n−3 fatty acids from fatty fish - also tend to have higher proportions of n−3, increased HDL cholesterol and decreased triglycerides (fatty material that circulates in the blood) and less heart disease. Eating walnuts (the ratio of n−3 to n−6 is circa 1:4 respectively[57]) was reported to lower total cholesterol by 4% relative to controls when people also ate 27% less cholesterol.[58]
A study carried out involving 465 women showed serum levels of eicosapentaenoic acid is inversely related to the levels of anti-oxidized-LDL antibodies. Oxidative modification of LDL is thought to play an important role in the development of atherosclerosis.[59]
Immune function
Another study regarding fish oil was published in the Journal of Nutrition in April 2007. Sixty four healthy Danish infants from nine to twelve months of age received either cow's milk or infant formula alone or with fish oil. It was found that those infants supplemented with fish oil had improvement in immune function maturation with no apparent reduction in immune activation.[60]
Brain health
Fish oil may help prevent psychotic disorders in high-risk children and adolescents.[61] A novel fish oil known as E-EPA may prevent memory impairment[62] and speed up recovery from major depression[63] There was yet another study on n−3 fatty acids published in the April 2007 Journal of Neuroscience. A group of mice were genetically modified to develop accumulation of amyloid and tau proteins in the brain similar to that seen in people with poor memory. The mice were divided into four groups with one group receiving a typical American diet (with high ratio of n−6 to n−3 fatty acids being 10 to 1). The other three groups were given food with a balanced 1 to 1 n−6 to n−3 ratio and two additional groups supplemented with DHA plus long chain n−6 fatty acids. After three months of feeding, all the DHA supplemented groups were noted to have a lower accumulation of beta amyloid and tau protein. Some research suggests that these abnormal proteins may contribute to the development of memory loss in later years.[64]
There is also a study published regarding n−3 supplementation in children with learning and behavioral problems. This study was published in the April 2007 edition of the Journal of the Developmental and Behavioral Pediatrics (5), where 132 children, between the ages of seven to twelve years old, with poor learning, participated in a randomized, placebo-controlled, double-blinded interventional trial. A total of 104 children completed the trial. For the first fifteen weeks of this study, the children were given polyunsaturated fatty acids (n−3 and n−6, 3000 mg a day), polyunsaturated fatty acids plus multi-vitamins and minerals or placebo. After fifteen weeks, all groups crossed over to the polyunsaturated fatty acids (PUFA) plus vitamins and mineral supplement. Parents were asked to rate their children's condition after fifteen and thirty weeks. After thirty weeks, parental ratings of behavior improved significantly in nine out of fourteen scales. The lead author of the study, Dr. Sinn, indicated the present study is the largest PUFA trial to date with children falling in the poor learning and focus range. The results support those of other studies that have found improvement in poor developmental health with essential fatty acid supplementation.[55][56][60][64][65][66] After completion of Dr. Nathalie Sinn's study, positive results still continued and children's behaviors improved.[67]
A study[68] examining whether omega-3 exerts neuroprotective action in Parkinson's disease found that it did, using an experimental model, exhibit a protective effect (much like it did for Alzheimer's disease as well). The scientists exposed mice to either a control or a high omega-3 diet from two to twelve months of age and then treated them with a neurotoxin commonly used as an experimental model for Parkinson's. The scientists found that high doses of omega-3 given to the experimental group completely prevented the neurotoxin-induced decrease of dopamine that ordinarily occurs. Since Parkinson's is a disease caused by disruption of the dopamine system, this protective effect exhibited could show promise for future research in the prevention of Parkinson's disease.
However, fish oil has been shown to have no effect on cognitive performance in older individuals without dementia.[69]
Anti-inflammatory
Research in 2005 and 2006 has suggested that the in-vitro anti-inflammatory activity of n−3 acids translates into clinical benefits. Cohorts of neck pain patients and of rheumatoid arthritis sufferers have demonstrated benefits comparable to those receiving standard NSAIDs.
Risks
Health risks
Non-cardiac health risks
In a letter published October 31, 2000,[70] the United States Food and Drug Administration Center for Food Safety and Applied Nutrition, Office of Nutritional Products, Labeling, and Dietary Supplements noted that known or suspected risks of EPA and DHA n−3 fatty acids consumed in excess of 3 grams per day may include the possibility of:
- Increased incidence of bleeding.
- Hemorrhagic stroke.
- Oxidation of omega-3 fatty acids forming biologically active oxidation products.
- Increased levels of low density lipoproteins (LDL) cholesterol or apoproteins associated with LDL cholesterol among diabetics and hyperlipidemics.
- Reduced glycemic control among diabetics.
Subsequent advice from the FDA and national counterparts have permitted health claims associated with heart health.
Cardiac risk
Persons with congestive heart failure, chronic recurrent angina pectoris or evidence that their heart is receiving insufficient blood flow are advised to talk to their doctor before taking n−3 fatty acids. There have been concerns if such persons take n−3 fatty acids or eating foods that contain them in substantial amounts.[71] In a recent large study, n−3 fatty acids on top of standard heart failure therapy produced a small but statistically significant benefit in terms of mortality and hospitalization.[72]
In congestive heart failure, cells that are only barely receiving enough blood flow become electrically hyperexcitable. This, in turn, can lead to increased risk of irregular heartbeats, which, in turn, can cause sudden cardiac death. n−3 fatty acids seem to stabilize the rhythm of the heart by effectively preventing these hyperexcitable cells from functioning, thereby reducing the likelihood of irregular heartbeats and sudden cardiac death. For most people, this is obviously beneficial and would account for most of the large reduction in the likelihood of sudden cardiac death. Nevertheless, for people with congestive heart failure, the heart is barely pumping blood well enough to keep them alive. In these patients, n−3 fatty acids may eliminate enough of these few pumping cells that the heart would no longer be able to pump sufficient blood to live, causing an increased risk of cardiac death.[71]
Research frontiers
Developmental differences
Although not supported by current scientific evidence as a primary treatment for ADHD, autism, and other developmental differences,[73][74] omega-3 fatty acids have gained popularity for children with these conditions.[73] A 2004 Internet survey found that 29% of surveyed parents used essential fatty acid supplements to treat children with autism spectrum disorders.[75]
Omega-3 fatty acids offer a promising complementary approach to standard treatments for ADHD and developmental coordination disorder.[74] Fish oils appear to reduce ADHD-related symptoms in some children.[74] Double blind studies have showed "medium to strong treatment effects of omega 3 fatty acids on symptoms of ADHD" after administering amounts around 1 gram for three to six months.[65][76][77]
There is very little scientific evidence supporting the effectiveness of omega-3 fatty acids for autism spectrum disorders.[78] One randomized controlled trial found that omega-3 fatty acids did not significantly affect aberrant behavior in autistic children, and although the investigators noted reduced hyperactivity,[79] their later reanalysis reported that the reduction was not statistically significant.[80]
Low birth weight
In a study of nearly 9,000 pregnant women, researchers found women who ate fish once a week during their first trimester had 3.6 times less risk of low birth weight and premature birth than those who ate no fish. Low consumption of fish was a strong risk factor for preterm delivery and low birth weight.[81][82] However, attempts by other groups to reverse this increased risk by encouraging increased pre-natal consumption of fish were unsuccessful.[83]
Psychiatric disorders
n−3 fatty acids are thought by some to have membrane-enhancing capabilities in brain cells. One medical explanation is that n−3 fatty acids play a role in the fortification of the myelin sheaths. Not coincidentally, n−3 fatty acids comprise approximately eight percent of the average human brain according to Dr. David Horrobin, a pioneer in fatty acid research. Ralph Holman of the University of Minnesota, another major researcher in studying essential fatty acids, who gave Omega-3 its name, surmised how n−3 components are analogous to the human brain by stating that "DHA is structure, EPA is function."
A benefit of n−3 fatty acids is helping the brain to repair damage by promoting neuronal growth.[51] In a six-month study involving people with schizophrenia and Huntington's disease who were treated with E-EPA or a placebo, the placebo group had clearly lost cerebral tissue, while the patients given the supplements had a significant increase of grey and white matter.[84]
In the prefrontal cortex (PFC) of the brain, low brain n−3 fatty acids are thought to lower the dopaminergic neurotransmission in this brain area, possibly contributing to the negative and neurocognitive symptoms in schizophrenia. This reduction in dopamine system function in the PFC may lead to an overactivity in dopaminergic function in the limbic system of the brain which is suppressively controlled by the PFC dopamine system, causing the positive symptoms of schizophrenia. This is called the n−3 polyunsaturated fatty acid/dopamine hypothesis of schizophrenia (Ohara, 2007). This mechanism may explain why n−3 supplementation shows effects against both positive, negative and neurocognitive symptoms in schizophrenia.
Consequently, the past decade of n−3 fatty acid research has procured some Western interest in n−3 fatty acids as being a legitimate 'brain food.' Still, recent claims that one's intelligence quotient, psychological tests measuring certain cognitive skills, including numerical and verbal reasoning skills, are increased on account of n−3 fatty acids consumed by pregnant mothers remain unreliable and controversial. An even more significant focus of research, however, lies in the role of n−3 fatty acids as a non-prescription treatment for certain psychiatric and mental diagnoses and has become a topic of much research and speculation.
In 1998, Andrew L. Stoll, MD and his colleagues at Harvard University conducted a small double-blind placebo-controlled study in thirty patients diagnosed with bipolar disorder. Most subjects in this study were already undergoing psychopharmacological treatment (e.g. 12 out of the 30 were taking lithium). Over the course of four months, he gave 15 subjects capsules containing olive oil, and another 15 subjects capsules containing nine grams of pharmaceutical-quality EPA and DHA. The study showed that subjects in the n−3 group were less likely to experience a relapse of symptoms in the four months of the study. Moreover, the n−3 group experienced significantly more recovery than the placebo group. However, a commentary on the Stoll study notes that the improvement in the n−3 group was too small to be clinically significant.[85] Though Stoll believes that the 1999 experiment was not as optimal as it could have been and has accordingly pursued further research, the foundation has been laid for more researchers to explore the theoretical association between absorbed n−3 fatty acids and signal transduction inhibition in the brain.[86]
"Several epidemiological studies suggest covariation between seafood consumption and rates of mood disorders. Biological marker studies indicate deficits in omega−3 fatty acids in people with depressive disorders, while several treatment studies indicate therapeutic benefits from omega-3 supplementation. A similar contribution of omega-3 fatty acids to coronary artery disease may explain the well-described links between coronary artery disease and depression. Deficits in omega-3 fatty acids have been identified as a contributing factor to mood disorders and offer a potential rational treatment approach."[87] In 2004, a study found that 100 suicide attempt patients on average had significantly lower levels of EPA in their blood as compared to controls.[88]
In 2006 the Omega-3 Fatty Acids Subcommittee, assembled by the Committee on Research on Psychiatric Treatments of the American Psychiatric Association (APA) stated the following: "The preponderance of epidemiologic and tissue compositional studies supports a protective effect of omega-3 EFA intake, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), in mood disorders. Meta-analyses of randomized controlled trials demonstrate a statistically significant benefit in unipolar and bipolar depression (p=.02). The results were highly heterogeneous, indicating that it is important to examine the characteristics of each individual study to note the differences in design and execution. There is less evidence of benefit in schizophrenia. EPA and DHA appear to have negligible risks and some potential benefit in major depressive disorder and bipolar disorder, but results remain inconclusive in most areas of interest in psychiatry. Health benefits of omega-3 EFA may be especially important in patients with psychiatric disorders, due to high prevalence rates of smoking and obesity and the metabolic side effects of some psychotropic medications." [89]
Another meta-analysis published in the Journal of Clinical Psychiatry in 2007, based on 10 clinical trials, found that Omega-3 polyunsaturated fatty acids significantly improved depression in patients with both unipolar and bipolar disorder. However, based upon the heterogeneity of the trials, the authors concluded that "more large-scale, well-controlled trials are needed to find out the favorable target subjects, therapeutic dose of EPA and the composition of omega-3 PUFAs in treating depression".[90] A small American trial, published in 2009, suggests that E-EPA, as monotherapy, has an advantage over placebo in major depressive disorder.[91]
Dietary sources
Daily values
As macronutrients, fats are not assigned recommended daily allowances. Macronutrients have AI (acceptable intake) and AMDR (acceptable macronutrient distribution range) instead of RDAs. The AI for n−3 is 1.6 grams/day for men and 1.1 grams/day for women[92] while the AMDR is 0.6% to 1.2% of total energy.[93]
A growing body of literature suggests that higher intakes of α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) may afford some degree of protection against coronary heart disease. Because the physiological potency of EPA and DHA is much greater than that for α-linolenic acid, it is not possible to estimate one AMDR for all n−3 fatty acids. Approximately 10 percent of the AMDR can be consumed as EPA and/or DHA."[93] There was insufficient evidence as of 2005 to set a UL (upper tolerable limit) for n−3 fatty acids.[92]
A perceived risk of fish oil n−3 supplementation has been heavy metal poisoning by the body's accumulation of traces of heavy metals, in particular mercury, lead, nickel, arsenic and cadmium as well as other contaminants (PCBs, furans, dioxins, PBDEs), which potentially might be found especially in less-refined fish oil supplements. However, in reality, heavy metal toxicity from consuming fish oil supplements is highly unlikely. This is because heavy metals selectively bind with protein in the fish flesh rather than accumulate in the oil. An independent test in 2006 of 44 fish oils on the US market found that all of the products passed safety standards for potential contaminants.[94] The FDA recommends that total dietary intake of n−3 fatty acids from fish not exceed 3 grams per day, of which no more than 2 grams per day are from nutritional supplements.[5]
Historically, the Council for Responsible Nutrition (CRN) and the World Health Organization (WHO) have published acceptable standards regarding contaminants in fish oil. The most stringent current standard is the International Fish Oils Standard (IFOS). The Global Organization for EPA and DHA Omega-3 (GOED)[95] has also published standards for omega-3 products. Fish oils that typically make this highest grade are those that are molecularly distilled under vacuum, and have virtually no measurable level of contaminants (measured parts per billion and parts per trillion).
n−3 supplementation in food has been a significant recent trend in food fortification, with global food companies launching n−3 fortified bread, mayonnaise, pizza, yogurt, orange juice, children's pasta, milk, eggs, confections and infant formula.
Fish
The most widely available source of EPA and DHA is cold water oily fish such as salmon, herring, mackerel, anchovies and sardines. Oils from these fish have a profile of around seven times as much n−3 as n−6. Other oily fish such as tuna also contain n−3 in somewhat lesser amounts. Consumers of oily fish should be aware of the potential presence of heavy metals and fat-soluble pollutants like PCBs and dioxin which may accumulate up the food chain. After extensive review, researchers from Harvard's School of Public Health reported in the Journal of the American Medical Association (2006) that the benefits of fish intake generally far outweigh the potential risks. As fish oil supplements are bought for their healthful Omega-3 fatty acid content, it is therefore vital that manufacturers and suppliers of these products ensure that they do not contain high levels of dioxins and other toxins.[96]
Even some forms of fish oil may not be optimally digestible. Of four studies that compare bioavailability of the triglyceride form of fish oil vs. the ester form, two have concluded that the natural triglyceride form is better, and the other two studies did not find a significant difference. No studies have shown the ester form to be superior although it is cheaper to manufacture.[97][98]
Although fish is a dietary source of n−3 fatty acids, fish do not synthesize them; they obtain them from the algae (microalgae in particular) or plankton in their diet.[99]
| Common name | grams n−3 |
|---|---|
| Tuna | 0.21–1.1 |
| Pollock | 0.45 |
| Salmon | 1.1–1.9 |
| Cod | 0.15–0.24 |
| Catfish | 0.22–0.3 |
| Flounder | 0.48 |
| Grouper | 0.23 |
| Halibut | 0.60–1.12 |
| Mahi mahi | 0.13 |
| Orange roughy | 0.028 |
| Red snapper | 0.29 |
| Shark | 0.83 |
| Swordfish | 0.97 |
| Tilefish | 0.90 |
| King mackerel | 0.36 |
Krill
Krill oil is a relatively new source of n−3 fatty acids. Various claims are made in support of krill oil as a superior source of n−3 fatty acids, such as that krill are not susceptible to contamination like fish and contain a special antioxidant called astaxanthin. However, numerous studies have found krill is often contaminated by pollution and astaxanthin hasn't been demonstrated to have a very potent antioxidant capacity.[101][102]
Green-lipped mussel
Green-lipped mussel from New Zealand also known as Perna canaliculus is another source of n-3 fatty acids. Research suggests that green-lipped mussels contain a distinct blend of n-3 fatty acids in comparison to other sources of n-3s. Most published studies report green-lipped mussels’ health benefits with inflammation. The book The Inflammation Revolution by George Halpern, MD., PhD., professor at Hong Kong Polytechnic University discusses the effects of green-lipped mussels in comparison to NSAIDs in the treatment of inflammatory conditions, particularly arthritis.[103] Lyprinol is a patented New Zealand mussel oil extract.[104]
Botanical sources

Six times richer than most fish oils in n−3,[105] albeit in the short chain form lacking EPA and DHA, flax (or linseed) (Linum usitatissimum) and its oil are perhaps the most widely available botanical source of n−3. Flaxseed oil consists of approximately 55% ALA (alpha-linolenic acid). Flax, like chia, contains approximately three times as much n−3 as n−6.
Purslane contains more Omega-3 fatty acids (alpha-linolenic acid in particular[106]) than any other leafy vegetable plant. Purslane has .01 mg/g of Eicosapentaenoic acid (EPA); this is an extraordinary amount of EPA for vegetable sources.
Table 1. ALA content as the percentage of n−3 in the seed oil.[107]
| Common name | Alternative name | Linnaean name | % n−3 |
|---|---|---|---|
| Chia | chia sage | Salvia hispanica | 64 |
| Kiwifruit | Chinese gooseberry | Actinidia chinensis | 62 |
| Perilla | shiso | Perilla frutescens | 58 |
| Flax | linseed | Linum usitatissimum | 55 |
| Lingonberry | Cowberry | Vaccinium vitis-idaea | 49 |
| Camelina | Gold-of-pleasure | Camelina sativa | 36 |
| Purslane | Portulaca | Portulaca oleracea | 35 |
| Black Raspberry | Rubus occidentalis | 33 | |
| Hemp | Cannabis Sativa | 19 |
Table 2. ALA content as the percentage of n−3 in the whole food.[108][109]
| Common name | Linnaean name | % n−3 |
|---|---|---|
| Flaxseed | Linum usitatissimum | 18.1 |
| Butternuts | Juglans cinerea | 8.7 |
| Hempseed | Cannabis sativa | 8.7 |
| Walnuts | Juglans regia | 6.3 |
| Pecan nuts | Carya illinoinensis | 0.6 |
| Hazel nuts | Corylus avellana | 0.1 |
Eggs
Eggs produced by chicken fed a diet of greens and insects produce higher levels of n−3 fatty acids (mostly ALA) than chicken fed corn or soybeans.[110] In addition to feeding chickens insects and greens, fish oils may be added to their diet to increase the amount of fatty acid concentrations in eggs.[111] The addition of flax and canola seeds to the diet of chickens, both good sources of alpha-linolenic acid, increases the omega-3 content of the eggs.[112] However, the Center for Science in the Public Interest reports that "the omega-3s that FDA considers healthful (DHA and EPA) are not found in plants such as flax seed." It also reports that "Eggs contain too much saturated fat and cholesterol to meet FDA’s definition of healthy."[113] The addition of green algae or seaweed to the diet boosts the content of DHA and EPA omega-3 content, which are the forms of omega-3 that are approved by the FDA for medical claims.
Meat
The n−6 to n−3 ratio of grass-fed beef is about 2:1, making it a more useful source of n−3 than grain-fed beef, which usually has a ratio of 4:1.[114]
In most countries, commercially available lamb is typically grass-fed, and thus higher in n−3 than other grain-fed or grain-finished meat sources. In the United States, lamb is often finished (i.e. fattened before slaughter) with grain, resulting in lower n−3.[115]
The omega-3 content of chicken meat may be enhanced by increasing the animals' dietary intake of grains that are high in n−3, such as flax, chia, and canola.[116]
Kangaroo meat is also a source of n−3 with fillet and steak containing 74mg per 100g of raw meat.[117]
Seal oil
Seal oil is a source of EPA, DPH, and DPA. According to Health Canada, it helps to support the development of the brain, eyes and nerves in children up to 12 years of age.[118] However, like all seal products, it is not allowed for import into the European Union[119]
Other sources

Milk and cheese from grass-fed cows may also be good sources of n−3. One UK study showed that half a pint of milk provides 10% of the recommended daily intake (RDI) of ALA, while a piece of organic cheese the size of a matchbox may provide up to 88%".[120]
The microalgae Crypthecodinium cohnii and Schizochytrium are rich sources of DHA (22:6 n−3) and can be produced commercially in bioreactors. This is the only source of DHA acceptable to vegans. Oil from brown algae (kelp) is a source of EPA. Walnuts are one of few nuts that contain appreciable n−3 fat, with approximately a 1:4 ratio of n−3 to n−6.[57] Acai palm fruit also contains n−3 fatty acids.
Omega-3 is also found in softgels in pharmacies and nowadays it is also found in combination with omega-6, omega-9 and shark liver oil [121]
The n−6 to n−3 ratio
Clinical studies[11][122][123] indicate that the ingested ratio of n−6 to n−3 (especially Linoleic vs Alpha Linolenic) fatty acids is important to maintaining cardiovascular health. However, two studies published in 2005 and 2007 found no such correlations in humans.[124][125]
Both n−3 and n−6 fatty acids are essential, i.e. humans must consume them in the diet. n−3 and n−6 compete for the same metabolic enzymes, thus the n−6:n−3 ratio will significantly influence the ratio of the ensuing eicosanoids (hormones), (e.g. prostaglandins, leukotrienes, thromboxanes etc.), and will alter the body's metabolic function.[126] Generally, grass-fed animals accumulate more n−3 than do grain-fed animals which accumulate relatively more n−6. Metabolites of n−6 are significantly more inflammatory (esp. arachidonic acid) than those of n−3. This necessitates that n−3 and n−6 be consumed in a balanced proportion; healthy ratios of n−6:n−3 range from 1:1 to 4:1.[127][128] Studies suggest that the evolutionary human diet, rich in game animals, seafood and other sources of n−3, may have provided such a ratio.[129][130]
Typical Western diets provide ratios of between 10:1 and 30:1 - i.e., dramatically skewed toward n−6.[131] Here are the ratios of n−6 to n−3 fatty acids in some common oils: canola 2:1, soybean 7:1, olive 3–13:1, sunflower (no n−3), flax 1:3,[132] cottonseed (almost no n−3), peanut (no n−3), grapeseed oil (almost no n−3) and corn oil 46 to 1 ratio of n−6 to n−3.[133]
Conversion efficiency of ALA to EPA and DHA
It has been reported that conversion of ALA to EPA and further to DHA in humans is limited, but varies with individuals.[134] Women have higher ALA conversion efficiency than men, probably due to the lower rate of utilization of dietary ALA for beta-oxidation. This suggests that biological engineering of ALA conversion efficiency is possible. Goyens et al. argue that it is the absolute amount of ALA, rather than the ratio of n−3 and n−6 fatty acids, which affects the conversion.[135]
See also
|
|
|
Notes and references
- ↑ Gerster H (1998). "Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)?". Int. J. Vitam. Nutr. Res. 68 (3): 159–173. PMID 9637947.
- ↑ Brenna JT (March 2002). "Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man.". Curr. Opin. Clin. Nutr. Metab. Care 5 (2): 127–132. doi:10.1097/00075197-200203000-00002. PMID 11844977.
- ↑ Burdge GC, Calder PC (September 2005). "Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults.". Reprod. Nutr. Dev. 45 (5): 581–597. doi:10.1051/rnd:2005047. PMID 16188209.
- ↑ Holman RT (February 1998). "The slow discovery of the importance of omega 3 essential fatty acids in human health". J. Nutr. 128 (2 Suppl): 427S–433S. PMID 9478042.
- ↑ 5.0 5.1 United States Food and Drug Administration (September 8, 2004). "FDA announces qualified health claims for omega-3 fatty acids". Press release. http://www.fda.gov/SiteIndex/ucm108351.htm. Retrieved 2006-07-10.
- ↑ Canadian Food Inspection Agency. Summary Table of Biological Role Claims Table 8-2. http://www.inspection.gc.ca/english/fssa/labeti/guide/ch8e.shtml
- ↑ Venturi S; Bégin ME (2010). "Thyroid Hormone, Iodine and Human Brain Evolution". In Cunnane S; Stewart K. Environmental Influences on Human Brain Evolution. John Wiley & Sons. pp. 105–124. ISBN 978-0-470-45268-4.
- ↑ Cocchi M. and Venturi S. Iodide, antioxidant function and Omega-6 and Omega-3 fatty acids: a new hypothesis of a biochemical cooperation? Progress in Nutrition, 2000, 2, 15-19
- ↑ Ingenbleek Y, et al.(1997). Iodised rapeseed oil for eradication of severe endemic goitre. Lancet. 1997, 22;350(9090):1542-5
- ↑ Ingenbleek Y, Jung L, Férard G, (2000). Brassiodol: A new iodised oil for eradication of endemic goitre. Journal of Trace Elements in Medicine and Biology. 2000, 13: 85-96
- ↑ 11.0 11.1 Lands, William E.M. (1 May 1992). "Biochemistry and physiology of n–3 fatty acids". FASEB Journal (Federation of American Societies for Experimental Biology) 6 (8): 2530–2536. PMID 1592205. http://www.fasebj.org/cgi/reprint/6/8/2530/. Retrieved 2008-03-21.
- ↑ Shearer GC, Harris WS, Pedersen TL, Newman JW. (August 2009) Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res. Full Free text
- ↑ von Schacky C. (March 2003). "The role of omega-3 fatty acids in cardiovascular disease.". Curr. Atheroscler. Rep. 5 (2): 139–45. doi:10.1007/s11883-003-0086-y. PMID 12573200.
- ↑ Morris, Martha C.; Sacks, Frank; Rosner, Bernard (1993). "Does fish oil lower blood pressure? A meta-analysis of controlled trials". Circulation 88 (2): 523–533. PMID 8339414. http://circ.ahajournals.org/cgi/reprint/88/2/523/.
- ↑ Mori, Trevor A.; Bao, Danny Q.; Burke, Valerie; Puddey, Ian B.; Beilin, Lawrence J. (1993). "Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans". Hypertension 34 (2): 253–260. PMID 10454450. http://hyper.ahajournals.org/cgi/reprint/34/2/253/.
- ↑ Harris, William S. (1997). "n−3 fatty acids and serum lipoproteins: human studies". Am J Clin Nutr 65 (5 Sup.): 1645S–1654S. PMID 9129504. http://www.ajcn.org/cgi/reprint/65/5/1645S/.
- ↑ Sanders, T.A.B.; Oakley, F.R.; Miller, G.J.; Mitropoulos, K.A.; Crook, D.; Oliver, M.F. (1997). "Influence of n−6 versus n−3 polyunsaturated fatty acids in diets low in saturated fatty acids on plasma lipoproteins and hemostatic factors". Arteriosclerosis, Thrombosis, and Vascular Biology 17 (12): 3449–3460. PMID 9437192. http://atvb.ahajournals.org/cgi/content/full/17/12/3449.
- ↑ Roche, H.M.; Gibney, M.J. (1996). "Postprandial triacylglycerolaemia: the effect of low-fat dietary treatment with and without fish oil supplementation". Eur J Clin Nutr. 50 (9): 617–624. PMID 8880041. http://cat.inist.fr/?aModele=afficheN&cpsidt=3232572.
- ↑ Davidson MH, Stein EA, Bays HE, Maki KC, Doyle RT, Shalwitz RA, Ballantyne CM, Ginsberg HN (2007). "Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to Simvastatin 40 mg/d in hypertriglyceridemic patients: An 8-week, randomized, double-blind, placebo-controlled study". Clin Ther. 29 (7): 1354–1367. doi:10.1016/j.clinthera.2007.07.018. PMID 17825687.
- ↑ Bucher HC, Hengstler P, Schindler C, Meier G. (2002). "n−3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials". Am J Med 112 (4): 298–304. doi:10.1016/S0002-9343(01)01114-7. PMID 11893369.
- ↑ Burr, Michael L.; Sweetham, P.M.; Fehily, Ann M. (August 1994). "Diet and reinfarction". European Heart Journal 15 (8): 1152–1153. PMID 7988613. http://eurheartj.oxfordjournals.org/cgi/reprint/15/8/1152.
- ↑ Willett, Walter C.; Stampfer, M.J.; Colditz, G.A.; Speizer, F.E.; Rosner, B.A.; Hennekens, C.H. (1993). "Intake of trans fatty acids and risk of coronary heart disease among women". The Lancet 341 (8845): 581–585. doi:10.1016/0140-6736(93)90350-P. PMID 8094827.
- ↑ Stone, Neil J. (1996). "Fish consumption, fish oil, lipids, and coronary heart disease". Circulation 94 (9): 2337–2340. PMID 8901708. http://circ.ahajournals.org/cgi/content/full/94/9/2337.
- ↑ Fortin PR, Lew RA, Liang MH, Wright EA, Beckett LA, Chalmers TC, Sperling RI. (1995). "Validation of a meta-analysis: The effects of fish oil in rheumatoid arthritis". J Clin Epidemiol 48 (11): 1379–1390. doi:10.1016/0895-4356(95)00028-3. PMID 7490601.
- ↑ Kremer, Joel M.; Bigauoette, J.; Michalek, A.V.; Timchalk, M.A.; Lininger, L.; Rynes, R.I.; Huyck, C.; Zieminski, J.; Bartholomew, L.E. (1985). "Effects of manipulation of dietary fatty acids on clinical manifestations of rheumatoid arthritis.". The Lancet (Elsevier) 1 (8422): 184–187. doi:10.1016/S0140-6736(85)92024-0. PMID 2857265.
- ↑ Christensen, Jeppe H.; Gustenhoff, Peter; Ejlersen, Ejler; Jessen, Torben; Korup, Eva; Rasmussen, Klaus; Dyerberg, Jørn; Schmidt, Erik B. (January 1995). "n−3 fatty acids and ventricular extrasystoles in patients with ventricular tachyarrhythmias". Nutrition Research 15 (1): 1–8. doi:10.1016/0271-5317(95)91647-U.
- ↑ Christensen, Jeppe H.; Gustenhoff, Peter; Korup, Eva; Aarøe, Jens; Toft, Egon; Møller, Torn; Rasmussen, Klaus; Dyerberg, Jørn; Schmidt, Erik B. (1996-03-16). "Effect of fish oil on heart rate variability in survivors of myocardial infarction: a double blind randomised controlled trial.". BMJ 312 (7032): 677–678. PMID 8597736. PMC 2350515. http://www.bmj.com/cgi/content/full/312/7032/677.
- ↑ Pignier, C.; Revenaz, C.; Rauly-Lestienne, I.; Cussac, D.; Delhon, A.; Gardette, J.; Le Grand, B. (2007). "Direct protective effects of poly-unsaturated fatty acids, DHA and EPA, against activation of cardiac late sodium current". Basic Research in Cardiology (Steinkopff Verlag) 102 (6): 553–564. doi:10.1007/s00395-007-0676-x. PMID 17891522. http://www.springerlink.com/content/u454873774830225/.
- ↑ 29.0 29.1 Su, Kuan-Pin; Huang, Shih-Yi; Chiub, Chih-Chiang; Shenc, Winston W. (2003). "Omega-3 fatty acids in major depressive disorder: A preliminary double-blind, placebo-controlled trial". Eur Neuropsychopharmacol 13 (4): 267–271. doi:10.1016/S0924-977X(03)00032-4. PMID 12888186.
- ↑ Naliwaiko, K.; Araújo, R.L.; da Fonseca, R.V.; Castilho, J.C.; Andreatini, R.; Bellissimo, M.I.; Oliveira, B.H.; Martins, E.F.; Curi, R.; Fernandes, L.C.; Ferraz, A.C. (April 2004). "Effects of fish oil on the central nervous system: a new potential antidepressant?". Nutritional Neuroscience (Maney) 7 (2): 91–99. doi:10.1080/10284150410001704525. PMID 15279495.
- ↑ Green, Pnina; Hermesh, Haggai; Monselisec, Assaf; Maromb, Sofi; Presburgerb, Gadi; Weizman, Abraham (2006). "Red cell membrane omega-3 fatty acids are decreased in nondepressed patients with social anxiety disorder". Eur Neuropsychopharmacol 16 (2): 107–113. doi:10.1016/j.euroneuro.2005.07.005. PMID 16243493.
- ↑ Yehuda S., Rabinovitz S., Mostofsky D.I. (2005). "Mixture of essential fatty acids lowers test anxiety". Nutritional Neuroscience 8 (4): 265–267. doi:10.1080/10284150500445795. PMID 16491653.
- ↑ Nemets, Boris; Stahl, Ziva; Belmaker, R.H. (2002). "Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder". Am J Psychiatry 159 (3): 477–479. doi:10.1176/appi.ajp.159.3.477. PMID 11870016.
- ↑ Caryn Rabin, Roni (October 26, 2009). "Regimens: Omega-3 Fats Fail to Lift Depression in Heart Patients". The New York Times. http://www.nytimes.com/2009/10/27/health/research/27regimens.html.
- ↑ Keli, S.O.; Feskens, E.J.; Kromhout, D. (1994). "Fish consumption and risk of stroke: The Zutphen Study". Stroke 25 (2): 328–332. PMID 8303739.
- ↑ Gillum, R.F.; Mussolino, M.E.; Madans, J.H. (1996). "The relationship between fish consumption and stroke incidence: The NHANES I Epidemiologic Follow-up Study (National Health and Nutrition Examination Survey)". Arch Intern Med 156 (5): 537–542. doi:10.1001/archinte.156.5.537. PMID 8604960.
- ↑ 37.0 37.1 Iso, H.; Rexrode, K.M.; Stampfer, M.J.; Manson, J.E.; Colditz, G.A.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. (2001). "Intake of fish and omega-3 fatty acids and risk of stroke in women". JAMA 285 (3): 304–312. doi:10.1001/jama.285.3.304. PMID 11176840.
- ↑ The U.S. Food and Drug Administration classification - GRAS (Generally Recognized as Safe)
- ↑ PMID 16825676 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Augustsson, Katarina; et al. (2003). "A prospective study of intake of fish and marine fatty acids and prostate cancer". Cancer Epidemiology, Biomarkers & Prevention 12 (1): 64–67. PMID 12540506.
- ↑ De Deckere, E.A. (1999). "Possible beneficial effect of fish and fish n−3 polyunsaturated fatty acids in breast and colorectal cancer". Eur J Cancer Prev 8 (3): 213–221. doi:10.1097/00008469-199906000-00009. PMID 10443950.
- ↑ Caygill, C.P.; Hill, M.J. (1995). "Fish, n−3 fatty acids and human colorectal and breast cancer mortality". Eur J Cancer Prev 4 (4): 329–332. doi:10.1097/00008469-199508000-00008. PMID 7549825.
- ↑ Yong Q. Chen, et al. (2007). "Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids". J Clin Invest 117 (7): 1866–75. doi:10.1172/JCI31494. PMID 1890998. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1890998. Retrieved 2008-11-30.
- ↑ Pala V, et al. (2001). "Erythrocyte Membrane Fatty Acids and Subsequent Breast Cancer: a Prospective Italian Study". JNCL 93 (14): 1088. doi:10.1093/jnci/93.14.1088. PMID 11459870. http://jnci.oxfordjournals.org/cgi/content/full/93/14/1088. Retrieved 2008-11-30.
- ↑ MacLean, Catherine H. et al. (2006). "Effects of n−3 Fatty Acids on Cancer Risk". JAMA 295 (4): 403–415. doi:10.1001/jama.295.4.403. PMID 16434631. http://jama.ama-assn.org/cgi/content/short/295/4/403. Retrieved 2006-07-07.
- ↑ Lee Hooper et al. (2006). "Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review". BMJ 332 (7544): 752–760. doi:10.1136/bmj.38755.366331.2F. PMID 16565093. PMC 1420708. http://bmj.bmjjournals.com/cgi/reprint_abr/332/7544/752/. Retrieved 2006-07-07.
- ↑ Colomer R, Moreno-Nogueira JM, García-Luna PP, et al. (May 2007). "N-3 fatty acids, cancer and cachexia: a systematic review of the literature". Br. J. Nutr. 97 (5): 823–31. doi:10.1017/S000711450765795X. PMID 17408522.
- ↑ Ryan AM, Reynolds JV, Healy L, et al. (2009). "Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial". Ann. Surg. 249 (3): 355–63. doi:10.1097/SLA.0b013e31819a4789. PMID 19247018.
- ↑ "Dietary supplementation with n−3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial.". Lancet 354 (9177): 447–455. 1999. doi:10.1016/S0140-6736(99)07072-5. PMID 10465168.
- ↑ Marchioli R.; Barzi, F; Bomba, E; Chieffo, C; Di Gregorio, D; Di Mascio, R; Franzosi, MG; Geraci, E et al. (2002). "Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the GISSI-Prevenzione.". Circulation 105 (16): 1897–1903. doi:10.1161/01.CIR.0000014682.14181.F2. PMID 11997274.
- ↑ 51.0 51.1 Trivedi, Bijal (2006-09-23). "The good, the fad, and the unhealthy". New Scientist: pp. 42–49. http://www.newscientist.com/channel/health/mg19125701.300-the-good-the-fad-and-the-unhealthy.html.
- ↑ Wang, C; Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J (July 2006). "n−3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review". Am J Clin Nutr 84 (1): 5–17. PMID 16825676.
- ↑ Mozaffarian, Dariush; Rimm, Eric B. (October 2006). "Fish intake, contaminants, and human health: evaluating the risks and the benefits". JAMA 296 (15): 1885–1899. doi:10.1001/jama.296.15.1885. PMID 17047219.
- ↑ Mita, T; Watada H, Ogihara T, Nomiyama T, Ogawa O, Kinoshita J, Shimizu T, Hirose T, Tanaka Y, Kawamori R (2007). "Eicosapentaenoic acid reduces the progression of carotid intima-media thickness in patients with type 2 diabetes". Atherosclerosis 191 (1): 162–167. doi:10.1016/j.atherosclerosis.2006.03.005. PMID 16616147.
- ↑ 55.0 55.1 McKenney, James M.; Sica, Domenic (2007). "Prescription omega-3 fatty acids for the treatment of hypertriglyceridemia". Am J Health-Sys Pharm 64 (6): 595–605. doi:10.2146/ajhp060164. PMID 17353568.
- ↑ 56.0 56.1 Yokoyama, M; Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K (March 2007). "Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis". Lancet 369 (9567): 1090–1098. doi:10.1016/S0140-6736(07)60527-3. PMID 17398308.
- ↑ 57.0 57.1 "Nutrition Facts and Analysis for Nuts, Walnuts, English". NutritionData. http://www.nutritiondata.com/facts-C00001-01c20oc.html.
- ↑ Zambón, D.; Sabaté, J.; Muñoz, S.; Campero, B.; Casals, E.; Merlos, M.; Laguna, J.C.; Ros, E. (2000). "Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women: A randomized crossover trial". Annals of Internal Medicine 132 (7): 538–546. PMID 10744590.
- ↑ Garrido-Sánchez, L.; García-Fuentes, E.; Rojo-Martínez, G.; Cardona, F.; Soriguer, F.; Tinahones, F.J. (February 2008). "Inverse relation between levels of anti-oxidized-LDL antibodies and eicosapentanoic acid (EPA)". Br J Nut 22 (3): 1–5. doi:10.1017/S0007114508921723. PMID 18252023.
- ↑ 60.0 60.1 Damsgaard, Camilla T.; Lauritzen, Lotte; Kjær, Tanja M.R.; Holm, Puk M.I.; Fruekilde, Maj-Britt; Michaelsen, Kim F.; Frøkiær, Hanne (2007). "Fish oil supplementation modulates immune function in healthy infants". J Nutr 137 (4): 1031–1036. PMID 17374672. http://jn.nutrition.org/cgi/reprint/137/4/1031.
- ↑ Amminger GP, Schäfer M, Papageorgiou K, et al. (Jan 2010)Long-Chain -3 Fatty Acids for Indicated Prevention of Psychotic Disorders: A Randomized, Placebo-Controlled Trial. Arch Gen Psychiatry. 2010;67(2):146-154. Full Free Text
- ↑ Taepavarapruk P, Song C. (Dec 2009) "Reductions of acetylcholine release and nerve growth factor expression are correlated with memory impairment induced by interleukin-1beta administrations: effects of omega-3 fatty acid EPA treatment." J Neurochem. [1]
- ↑ Mischoulon D, Papakostas GI, Dording CM, et al. (Dec 2009) "A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder." Journal of Clinical Psychiatry. Free Full Text
- ↑ 64.0 64.1 Green, KN; Martinez-Coria H, Khashwji H, Hall EB, Yurko-Mauro KA, Ellis L, LaFerla FM (2007). "Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-β and tau pathology via a mechanism involving presenilin 1 levels". J Neuroscience 27 (16): 4385–4395. doi:10.1523/JNEUROSCI.0055-07.2007. PMID 17442823.
- ↑ 65.0 65.1 Sinn, Natalie; Bryan, Janet (April 2007). "Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD". J Dev Behav Pediatrics 28 (2): 82–91. doi:10.1097/01.DBP.0000267558.88457.a5. PMID 17435458.
- ↑ Lee, Duk-Hee; Lee, In-Kyu; Jin, Soo-Hee; Steffes, Michael; Jacobs, David R. (March 2007). "Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999-2002". Diabetes Care 30 (3): 622–628. doi:10.2337/dc06-2190. PMID 17327331.
- ↑ Sinn, Natalie. "Omega 3, Concentration and Hyperactivity" [2], Australia, retrieved on 2007-02
- ↑ Bousquet, M.; Saint-Pierre, M.; Julien, C.; Salem, N.; Cicchetti, F.; Calon, F. (April 2008). "Beneficial effects of dietary omega-3 polyunsaturated fatty acid on toxin-induced neuronal degeneration in an animal model of Parkinson's disease". Fed Am Soc Exper Bio J 22 (4): 1213–1225. doi:10.1096/fj.07-9677com. PMID 18032633.
- ↑ van de Rest, O.; Geleijnse, J. M.; Kok, F.J.; van Staveren, W.A.; Dullemeijer, C.; OldeRikkert, M.G.M.; Beekman, A. T.F.; de Groot, C. P.G.M. (August 2008). "Effect of fish oil on cognitive performance in older subjects". Neurology 71 (6): 430–438. doi:10.1212/01.wnl.0000324268.45138.86. PMID 18678826.
- ↑ Lewis, Christine J.. "Letter Regarding Dietary Supplement Health Claim for Omega-3 Fatty Acids and Coronary Heart Disease". http://www.fda.gov/ohrms/dockets/dockets/95s0316/95s-0316-Rpt0272-38-Appendix-D-Reference-F-FDA-vol205.pdf. and "Letter Regarding Dietary Supplement Health Claim for Omega-3 Fatty Acids and Coronary Heart Disease". U.S. Food and Drug Administration via Internet Archive. October 31, 2000. http://web.archive.org/web/20061217002249/http://vm.cfsan.fda.gov/~dms/ds-ltr11.html. Retrieved 2009-10-30.
- ↑ 71.0 71.1 Ornish, Dean (2006-05-02). "The Dark Side of Good Fats". Newsweek: p. 2. http://www.newsweek.com/id/137192. Retrieved 2008-06-14.
- ↑ Gissi-Hf Investigators; Tavazzi, L; Maggioni, AP; Marchioli, R; Barlera, S; Franzosi, MG; Latini, R; Lucci, D et al. (August 2008). "Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial". Lancet 372 (9645): 1223. doi:10.1016/S0140-6736(08)61239-8. PMID 18757090. http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(08)61239-8.
- ↑ 73.0 73.1 Levy, Susan E.; Hyman, Susan L. (2005). "Novel treatments for autistic spectrum disorders". Ment Retard Dev Disabil Res Rev 11 (2): 131–142. doi:10.1002/mrdd.20062. PMID 15977319.
- ↑ 74.0 74.1 74.2 Richardson, Alexandra J. (2006). "Omega-3 fatty acids in ADHD and related neurodevelopmental disorders". Int Rev Psychiatry 18 (2): 155–172. doi:10.1080/09540260600583031. PMID 16777670.
- ↑ Green, VA; Pituch KA, Itchon J, Choi A, O'Reilly M, Sigafoos J (2006). "Internet survey of treatments used by parents of children with autism". Res Dev Disabil 27 (1): 70–84. doi:10.1016/j.ridd.2004.12.002. PMID 15919178.
- ↑ Richardson, Alexandra J.; Montgomery, Paul (2005). "The Oxford-Durham study: a randomized, controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder". Pediatrics 115 (5): 1360–1366. doi:10.1542/peds.2004–2164 (inactive 2009-04-03). PMID 15867048.
- ↑ Johnson M, Ostlund S, Fransson G, Kadesjö B, Gillberg C. (2008 Apr 30). "Omega-3/Omega-6 Fatty Acids for Attention Deficit Hyperactivity Disorder: A Randomized Placebo-Controlled Trial in Children and Adolescents.". J Atten Disord 12 (5): 394–401. doi:10.1177/1087054708316261. PMID 18448859.
- ↑ Bent, Stephen; Bertoglio, Kiah; Hendren, Robert L. (March 2009). "Omega-3 fatty acids for autistic spectrum disorder: a systematic review". J Autism Dev Disord 39 (8): 1145–54. doi:10.1007/s10803-009-0724-5. PMID 19333748.
- ↑ Amminger, G. Paul; et al. (2007). "Omega-3 fatty acids supplementation in children with autism: a double-blind randomized, placebo-controlled pilot study". Biol Psychiatry 61 (4): 551–553. doi:10.1016/j.biopsych.2006.05.007. PMID 16920077.
- ↑ Gilbert, Donald L. (2008). "Regarding 'omega-3 fatty acids supplementation in children with autism: a double-blind randomized, placebo-controlled pilot study'". Biol Psychiatry 63 (2): e13. doi:10.1016/j.biopsych.2007.03.028. PMID 17555722. Author reply: Amminger, G. Paul; Harrigan, Susan M. (February 2008). "Reply". Biol Psychiatry 63 (2): e15. doi:10.1016/j.biopsych.2007.04.002.
- ↑ Olsen, Sjúrður Fróði; Secher, Niels Jørgen (2002-02-23). "Low consumption of seafood in early pregnancy as a risk factor for preterm delivery: prospective cohort study". BMJ (Clinical Research Ed.) 324 (7335): 447. doi:10.1136/bmj.324.7335.447. PMID 11859044.
- ↑ Jensen, Craig L (2006). "Effects of n-3 fatty acids during pregnancy and lactation". Am J Clin Nutr 83 (6): 1452–1457. ISSN 0002-9165. http://www.ajcn.org/cgi/reprint/83/6/S1452.pdf.
- ↑ Odent, Michel; Colson, Suzanne; De Reu, Paul (2002-05-25). "Consumption of seafood and preterm delivery. Encouraging pregnant women to eat fish did not show effect". BMJ (Clinical Research Ed.) 324 (7348): 1279. PMID 12028992.
- ↑ Puri, Basant K. (2006). "High-resolution magnetic resonance imaging sinc-interpolation-based subvoxel registration and semi-automated quantitative lateral ventricular morphology employing threshold computation and binary image creation in the study of fatty acid interventions in schizophrenia, depression, chronic fatigue syndrome and Huntington's disease". Int Rev Psychiatry 18 (2): 149–154. doi:10.1080/09540260600583015. PMID 16777669.
- ↑ Calabrese, J.R.; Rapport, D.J.; Shelton, M.D. (1999). "Fish oils and bipolar disorder: A promising but untested treatment". Arch Gen Psychiatry 56 (5): 413–414; discussion 415–416. doi:10.1001/archpsyc.56.5.413. PMID 10232295.
- ↑ Stoll, A.L.; et al. (1999). "Omega 3 fatty acids in bipolar disorder: A preliminary double-blind, placebo-controlled trial". Arch Gen Psychiatry 56 (5): 407–412. doi:10.1001/archpsyc.56.5.407. PMID 10232294.
- ↑ Nemets, H.; Nemets, B.; Apter, A.; Bracha, Z.; Belmaker, R.H. (2006). "Omega-3 treatment of childhood depression: A controlled, double-blind pilot study". Am J Psychiatry 163 (6): 1098–1100. doi:10.1176/appi.ajp.163.6.1098. PMID 16741212.
- ↑ Huan, M.; et al. (2004). "Suicide attempt and n−3 fatty acid levels in red blood cells: a case control study in China". Biol Psychiatry 56 (7): 490–496. doi:10.1016/j.biopsych.2004.06.028. PMID 15450784. http://www.journals.elsevierhealth.com/periodicals/bps/article/PIIS0006322304007061/abstract.
- ↑ Freeman MP, Hibbeln JR, Wisner KL, et al. (2006) Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67(12):1954-67. Free Full Text
- ↑ Lin, Pao-Yen; Kuan-Pin Su (July 2007). "A Meta-Analytic Review of Double-Blind, Placebo-Controlled Trials of Antidepressant Efficacy of Omega-3 Fatty Acids". J Clin Psychiatry 68 (7): 1056–1061. doi:10.4088/JCP.v68n0712. PMID 17685742. http://www.psychiatrist.com/privatepdf/2007/v68n07/.
- ↑ Mischoulon D, Papakostas GI, Dording CM, et al. "A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder." J Clin Psychiatry. 2009 Aug 25. Abstract
- ↑ 92.0 92.1 Food and Nutrition Board, Institute of Medicine of the National Academies (2005), pp.423
- ↑ 93.0 93.1 Food and Nutrition Board, Institute of Medicine of the National Academies (2005), pp.770
- ↑ "Product Review: Omega-3 Fatty Acids (EPA and DHA) from Fish/Marine Oils". ConsumerLab.com. 2005-03-15. http://www.consumerlab.com/results/omega3.asp. Retrieved 2007-08-14.
- ↑
- ↑ "Pollutants found in fish oil capsules". BBC News. 2002-04-06. http://news.bbc.co.uk/1/hi/health/1911312.stm. Retrieved 2010-01-06.
- ↑ Lawson, L.D.; Hughes, B.G. (1988). "Absorption of eicosapentaenoic acid and docosahexaenoic acid from fish oil triacylglycerols or fish oil ethyl esters co-ingested with a high-fat meal". Biochem Biophys Res Commun 156 (2): 960–963. doi:10.1016/S0006-291X(88)80937-9. PMID 2847723.
- ↑ Beckermann, B.; Beneke, M.; Seitz, I. (1990). "Comparative bioavailability of eicosapentaenoic acid and docasahexaenoic acid from triglycerides, free fatty acids and ethyl esters in volunteers" (in German). Arzneimittel-Forschung 40 (6): 700–704. PMID 2144420.
- ↑ Falk-Petersen, S., S.; et al. (1998). "Lipids and fatty acids in ice algae and phytoplankton from the Marginal Ice Zone in the Barents Sea". Polar Biology 20 (1): 41–47. doi:10.1007/s003000050274. ISSN 0722-4060. http://cat.inist.fr/?aModele=afficheN&cpsidt=2356641.
- ↑ "Fish, Levels of Mercury and Omega-3 Fatty Acids". http://www.americanheart.org/presenter.jhtml?identifier=3013797.
- ↑ Corsolini S; Covaci, A; Ademollo, N; Focardi, S; Schepens, P (March 2006). "Occurrence of organochlorine pesticides (OCPs) and their enantiomeric signatures, and concentrations of polybrominated diphenyl ethers (PBDEs) in the Adélie penguin food web, Antarctica.". Environ. Pollut. 140 (2): 371–382. doi:10.1016/j.envpol.2005.04.039. PMID 16183185.
- ↑ Covaci A; Voorspoels, S; Vetter, W; Gelbin, A; Jorens, PG; Blust, R; Neels, H (August 2007). "Anthropogenic and naturally occurring organobrominated compounds in fish oil dietary supplements.". Environ. Sci. Technol. 41 (15): 5237–5244. doi:10.1021/es070239g. PMID 17822085.
- ↑ Halpern, G.M., The Inflammation Revolution. Square City Publishers, New York 2005. ISBN 0757002838
- ↑ Lyprinol in Brief
- ↑ Bartram (1998), pp.271
- ↑ David Beaulieu. "Edible Landscaping With Purslane". About.com. http://landscaping.about.com/cs/weedsdiseases/a/purslane.htm.
- ↑ "Seed Oil Fatty Acids - SOFA Database Retrieval". http://sofa.bfel.de.
- ↑ DeFilippis, Andrew P.; Laurence S. Sperling. "Understanding omega-3's" (PDF). http://www.biovita.fi/suomi/pdf/understanding_omega3.pdf. Retrieved 21 October 2007.
- ↑ Wilkinson, Jennifer. "Nut Grower's Guide: The Complete Handbook for Producers and Hobbyists" (PDF). http://www.publish.csiro.au/samples/Nut%20Growers%20GuideSample.pdf. Retrieved 21 October 2007.
- ↑ "How Omega-6s Usurped Omega-3s In US Diet". http://www.medicalnewstoday.com/medicalnews.php?newsid=51575.
- ↑ Trebunová, A.; Vasko, L.; Svedová, M.; Kasteľ, R.; Tucková, M.; Mach, P. (July 2007). "The influence of omega-3 polyunsaturated fatty acids feeding on composition of fatty acids in fatty tissues and eggs of laying hens". Deutsche Tierärztliche Wochenschrift 114 (7): 275–279. PMID 17724936.
- ↑ Cherian, G. Effect of feeding full fat flax and canola seeds to laying hens on the fatty acids composition of eggs, embryos, and newly hatched chicks. http://www.fao.org/agris/search/display.do?f=./1991/v1717/US9138554.xml;US9138554
- ↑ http://www.cspinet.org/new/200706211.html
- ↑ "Omega-3/Omega-6 fatty acid content of Grass Fed Beef". http://www.csuchico.edu/agr/grsfdbef/health-benefits/ben-o3-o6.html.
- ↑ "Specially Labeled Lamb". http://www.sheep101.info/labeledlamb.html.
- ↑ Azcona, J.O., Schang, M.J., Garcia, P.T., Gallinger, C., R. Ayerza (h), and Coates, W. (2008). "Omega-3 enriched broiler meat: The influence of dietary alpha-linolenic omega-3 fatty acid sources on growth, performance and meat fatty acid composition". Canadian Journal of Animal Science, Ottawa, Ontario, Canada, 88:257-269.
- ↑ "Gourment Game - Amazing Nutrition Facts".
- ↑ http://www.hc-sc.gc.ca/dhp-mps/prodnatur/applications/licen-prod/monograph/mono_seal_oil_huile_phoque-eng.php
- ↑ European Parliament (9 November 2009). "MEPs adopt strict conditions for the placing on the market of seal products in the European Union". Hearings. European Parliament. http://www.europarl.europa.eu/sides/getDoc.do?pubRef=-//EP//TEXT+IM-PRESS+20090504IPR54952+0+DOC+XML+V0//EN. Retrieved 12 March 2010.
- ↑ "More Omega 3 in Organic Milk". http://www.vetscite.org/publish/items/001719/.
- ↑ "Omega-3 Omega-6 Omega-9 for weight management. A Cardiosterol approach by Alta Care Laboratoires". http://www.articlesbase.com/supplements-and-vitamins-articles/omega-369-for-weight-management-a-cardiosterol-approach-435636.html.
- ↑ Okuyama H (2001). "High n−6 to n−3 ratio of dietary fatty acids rather than serum cholesterol as a major risk factor for coronary heart disease.". Eur J Lipid Sci Technol 103: 418–422. doi:10.1002/1438-9312(200106)103:6<418::AID-EJLT418>3.0.CO;2-# (inactive 2010-03-17).
- ↑ Griffin BA (2008). "How relevant is the ratio of dietary n−6 to n−3 polyunsaturated fatty acids to cardiovascular disease risk? Evidence from the OPTILIP study". Curr. Opin. Lipidol. 19 (1): 57–62. doi:10.1097/MOL.0b013e3282f2e2a8. PMID 18196988.
- ↑ Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, Rimm EB., D; Ascherio, A; Hu, FB; Stampfer, MJ; Willett, WC; Siscovick, DS; Rimm, EB (2005). "Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men.". Circulation 111 (2): 157–64. doi:10.1161/01.CIR.0000152099.87287.83. PMID 15630029. PMC 1201401. http://circ.ahajournals.org/cgi/content/full/111/2/157.
- ↑ Willett WC, WC (2007). "The role of dietary n-6 fatty acids in the prevention of cardiovascular disease.". J Cardiovasc Med 8: Suppl 1:S42–5. doi:10.2459/01.JCM.0000289275.72556.13. PMID 17876199.
- ↑ Tribole, E.F.; Thompson, RL; Harrison, RA; Summerbell, CD; Ness, AR; Moore, HJ; Worthington, HV; Durrington, PN et al. (2006). "Excess Omega-6 Fats Thwart Health Benefits from Omega-3 Fats". BMJ 332 (7544): 752–760. doi:10.1136/bmj.38755.366331.2F. PMID 16565093. PMC 1420708. http://www.bmj.com/cgi/eletters/332/7544/752#130637. Retrieved 2008-03-23.
- ↑ Tribole, 2007
- ↑ Lands, 2005
- ↑ Simopoulos, AP (September 2003). "Importance of the ratio of omega-6/omega-3 essential fatty acids: evolutionary aspects". World Review of Nutrition and Dietetics 92: 1–174. doi:10.1159/000073788. ISBN 3805576404. PMID 14579680.
- ↑ Simopoulos AP, Leaf A, Salem Jr N (2000). "Statement on the essentiality of and recommended dietary intakes for n−6 and n−3 fatty acids". Prostaglandins Leukot Essent Fatty Acids 63 (63): 119–121. doi:10.1054/plef.2000.0176. PMID 10991764.
- ↑ Hibbeln et al., 2006
- ↑ Erasmus, Udo, Fats and Oils. 1986. Alive books, Vancouver, ISBN 0-920470-16-5 p. 263 (round-number ratio within ranges given.)
- ↑ "Essential Fats in Food Oils, NIH page". http://efaeducation.nih.gov/sig/esstable.html.
- ↑ "Conversion Efficiency of ALA to DHA in Humans". http://dhaomega3.org/index.php?category=overview&title=Conversion-of-ALA-to-DHA. Retrieved 21 October 2007.
- ↑ Goyens, Petra LL; et al. (1 Jul 2006). "Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio". American Journal of Clinical Nutrition 84 (1): 44. PMID 16825680. http://www.ajcn.org/cgi/content/abstract/84/1/44. Retrieved 21 October 2007.
Additional sources
- Bartram, Thomas, 1998, Bartram's Encyclopedia of Herbal Medicine, p. 271.
- Bell, J.G., et al. (2004). "Essential fatty acids and phospholipase A2 in autistic spectrum disorders." Prostaglandins Leukot.Essent.Fatty Acids 71(4):201–204. PMID 15301788
- Cunnane SC (2006) "Survival of the fattest: the key to human brain evolution." M S-MEDECINE SCIENCES 22 (6–7): 659–663.(article in French) PMID 16828044
- Food and Nutrition Board, Institute of Medicine of the National Academies (2005). Dietary Reference Intakes For Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press. ISBN 0-309-08537-3. http://newton.nap.edu/books/0309085373/html/770.html.
- Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE.(2006). "Healthy intakes of n−3 and n−6 fatty acids: estimations considering worldwide diversity". Am J Clin Nutr. 83(6 Suppl):1483S–1493S.
- Lands, William E.M. "Fish, Omega-3 and Human Health" Champaign. AOCS Press. 2005 ISBN 1-893997-81-2
- Ohara, K. (2007). "The n−3 polyunsaturated fatty acid/dopamine hypothesis of schizophrenia." Prog Neuropsychopharmacol Biol Psychiatry.31(2):469–474. PMID 17184889
- Richardson, A.J., and M.A. Ross. (2000). "Fatty acid metabolism in neurodevelopmental disorder: a new perspective on associations between attention-deficit/hyperactivity disorder, dyslexia, dyspraxia and the autistic spectrum." Prostaglandins Leukot.Essent.Fatty Acids 63(1–2):1–9. PMID 10970706
- Robson, A. (2006). "Shellfish view of omega-3 and sustainable fisheries." Nature 444, 1002.
- Robson, A. (2007). "Preventing the diseases of civilisation: shellfish, the omega-3:6 balance and human health." Shellfish News 23, 25–27
- Scher, J., and Pillinger, M. (2005) "15d-PGJ2: The anti-inflammatory prostaglandin?" Clinical Immunology. 114(2):100–109 PMID 15639643
- Tribole, Evelyn. "The Ultimate Omega-3 Diet" New York. McGraw-Hill. 2007 ISBN 13:978-0-07-146986-9
- Young, G., and J. Conquer. (2005). "Omega-3 fatty acids and neuropsychiatric disorders." Reprod.Nutr.Dev 45(1):1–28. PMID 15865053
Further reading
- Allport, Susan. The Queen of Fats: Why Omega-3s Were Removed from the Western Diet and What We Can Do to Replace Them. University of California Press, September 2006. ISBN 978-0-520-24282-1.
- Boyd, Hillary & Basant, Puri K. The Natural Way to Beat Depression: the groundbreaking discovery of EPA to change your life. London. Hodder and Stoughton. 2004. ISBN 0-340-82497-2
- Chow, Ching Kuang. Fatty Acids in Foods and Their Health Implications. Routledge Publishing. New York, New York. 2001.
- Clover, Charles. The End of the Line: How overfishing is changing the world and what we eat. Ebury Press, London 2004. ISBN 0-09-189780-7
- Erasmus, Udo. Fats That Heal, Fats That Kill. 3rd ed. Burnaby (BC): Alive Books; 1993.
- Smithers, Lois. The Food Industry's Greed. How Misleading Labeling of Omega-3 Foods Undermines American Health.
- Stoll, Andrew L. The Omega-3 Connection. Simon & Schuster 2001. ISBN 0-684-87138-6.
External links
- DHA/EPA Omega-3 Institute Organization founded to educate health professionals and the public about omega-3
- American Heart Assoc "Fish & Omega-3 Fatty Acids"
- BBC News report: Oily fish helps cut inflammation, March 12, 2005.
- University of Maryland Medical Center, omega-3 Fatty Acids
- Durham Research: Using Fatty Acids for Enhancing Classroom Achievement Website for the Durham Schools Trial, trial on the effects of fatty acids with children who were under performing in class. Funded by the Durham City Council and Oxford University. Their initial results (also available on durhamtrial.org) were published in May 2005.
- Essential (Omega-3 and Omega-6) Fatty Acids: The Linus Pauling Institute Micronutrient Information Center
- Simopoulos, AP The importance of the ratio of omega-6/omega-3 essential fatty acids.
- MedlinePlus Herbs and Supplements: Omega-3 fatty acids, fish oil, alpha-linolenic acid
- Another reason why men like curves (Daily Telegraph) Omega-3 credited with shaping a woman's figure to be more attractive to men.
- Long-Chain Polyunsaturated Fatty Acids in Human Brain Evolution. Crawford M.A. In: Environmental Influences on Human Brain Evolution. Cunnane, S. / Stewart, K. (eds.)
|
||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||
|
||||||||||||||||||||||||||||||